
#125 META-DOT
2,4-DIMETHOXY-5-METHYLTHIOAMPHETAMINE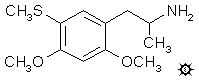
|
| [3D .mol structure] |
To a stirred and gently refluxing suspension of 11 g LAH in 750 mL anhydrous Et2O, there was added 13.2 g 2,4-dimethoxybenzenesulfonyl chloride in an Et2O solution. The refluxing was maintained for 48 h then, after cooling externally with ice water, the excess hydride was destroyed by the slow addition of 600 mL of 10% H2SO4. The phases were separated, and the aqueous phase extracted with 2x200 Et2O. The organics were pooled, washed once with 200 mL H2O, and the solvent removed under vacuum. The residue was dried azeotropically through the addition and subsequent removal of CH2Cl2. Distillation of the residue provided 8.0 g 2,4-dimethoxythiophenol as a colorless oil, boiling at 89-92 °C at 0.5 mm/Hg.
To a solution of 7.8 g 2,4-dimethoxythiophenol in 40 mL absolute EtOH there was added a solution of 4 g 85% KOH in 65 mL EtOH. This was followed by the addition of 5 mL methyl iodide, and the mixture was held at reflux for 30 min. This was poured into 200 mL H2O, and extracted with 3x50 mL Et2O. The pooled extracts were washed once with aqueous sodium hydrosulfite, then the organic solvent was removed under vacuum. The residue was distilled to give 8.0 g of 2,4-dimethoxythioanisole as a colorless oil with a bp of 100-103 °C at 0.6 mm/Hg.
To a mixture of 15 g POCl3 and 14 g N-methylformanilide that had been warmed briefly on the steam bath there was added 7.8 g of 2,4-dimethoxythioanisole. The reaction was heated on the steam bath for an additional 20 min and then poured into 200 mL H2O. Stirring was continued until the insolubles had become completely loose and granular. These were removed by filtration, washed with H2O, sucked as dry as possible, and then recrystallized from boiling MeOH. The product, 2,4-dimethoxy-5-(methylthio)benzaldehyde, was an off-white solid weighing 8.6 g. It could be obtained in either of two polymorphic forms, depending on the concentration of aldehyde in MeOH at the time of crystal appearance. One melted at 109-110 °C and had a fingerprint IR spectrum including peaks at 691, 734, 819 and 994 cm-1. The other melted at 124.5-125.5 °C and had major fingerprint peaks at 694, 731, 839 and 897 cm-1. Anal. (C10H12O3S) C,H.
A solution of 8.2 g 2,4-dimethoxy-5-(methylthio)benzaldehyde in 30 mL nitroethane was treated with 1.8 g anhydrous ammonium acetate and heated on the steam bath for 4 h. Removal of the excess nitroethane under vacuum gave a colored residue which crystallized when diluted with MeOH. Recrystallization of the crude product from boiling EtOH gave, after filtration, washing and air drying to constant weight, 8.3 g 1-(2,4-dimethoxy-5-methylthiophenyl)-2-nitropropene with a mp of 112-113 °C. Anal. (C12H15NO4S) C,H,N.
A suspension of 6.5 g LAH in 250 mL anhydrous THF was placed under a N2 atmosphere and stirred magnetically and brought to reflux. There was added, dropwise, 8.0 g of 1-(2,4-dimethoxy-5-methylthiophenyl)-2-nitropropene in 50 mL THF. The reaction mixture was maintained at reflux for 18 h. After being brought to room temperature, the excess hydride was destroyed by the addition of 6.5 mL H2O in 30 mL THF. There was then added 6.5 mL of 3N NaOH, followed by an additional 20 mL H2O. The loose, white, inorganic salts were removed by filtration, and the filter cake washed with an additional 50 mL THF. The combined filtrate and washes were stripped of solvent under vacuum yielding a residue that was distilled. The free base boiled at 125-128 °C at 0.1 mm/Hg and was a white oil which solidified on standing. It weighed 5.1 g and had a mp of 47-48.5 °C. This was dissolved in 50 mL IPA, neutralized with concentrated HCl (until dampened universal pH paper showed a deep red color) and diluted with anhydrous Et2O to the point of turbidity. There was a spontaneous crystallization providing, after filtering, washing with Et2O, and air drying, 2,4-dimethoxy-5-methylthioamphetamine hydrochloride (META-DOT) with a mp of 140.5-142 °C. Anal. (C12H20ClNO2S) C,H,N.
DOSAGE: greater than 35 mg.
DURATION: unknown.
QUALITATIVE COMMENTS: (with 35 mg) There was a vague awareness of something all afternoon, something that might be called a thinness. Possibly some brief cardiovascular stimulation, but nothing completely believable. This is a threshold level at the very most.
EXTENSIONS AND COMMENTARY: Again, as with the studies with ORTHO-DOT, it is apparent that the activity of META-DOT is going to be way down from the most interesting of these isomers, PARA-DOT (ALEPH-1, or just ALEPH). In the rectal hyperthermia assay (which calculates the psychedelic potential of compounds by seeing how they influence the body temperature of experimental animals in comparison to known psychedelics) the three DOT's were compared with DOM. And the results fell into line in keeping with the activities (or loss of activities) found in man. PARA-DOT was about half as active as DOM, but both ORTHO-DOT and the compound described here, META-DOT, were down by factors of 50x and 30x respectively. These animal studies certainly seem to give results that are reasonable with a view to other known psychedelic drugs, in that mescaline was down from DOM by a factor of more than 1000x, and LSD was some 33x more potent than DOM.
I have a somewhat jaundiced view of this rabbit rectal hyperthermia business. One is presumably able to tell whether a compound is a stimulant or a psychedelic drug by the profile of the temperature rise, and how potent it will be by the extent of the temperature rise. But the concept of pushing thermocouples into the rear ends of restrained rabbits somehow does not appeal to me. I would rather determine both of these parameters from human studies.
| [ |
[Main Index] | [Forward |

