Ayahuasca: alkaloids, plants & analogs
Section 3 : Part 2 :
Extreme-condition hostilis extraction
The following appeared in the 13 (2) 2004 Entheogen Review. It is reproduced with Pachano's permission.
as told to Mambo Pachano
Several interesting modifications to a normal isolation approach have proved effective in overcoming both the adverse impact of tannins and the emulsion forming nature commonly encountered in the extraction process when applied to Mimosa rootbark.
I do not know who to credit as this seems to have resulted from the interactive exploration of at least several people, mostly anonymous, over the course of the last two or three years.
The use of acidic extraction at pH 1 has been questioned as being too extreme and merely overkill but it is worth noting that this approach WORKS and these results have now been replicated by a growing number of people.
Critics can continue to say what they want but any opinions arising from an armchair viewpoint are worth a whole lot less than those resulting from some meaningful wet glove time (imho).
Thanks to all involved.
At least one person we know has reproduced the following process but switched methylene chloride for toluene and obtained the same end results. (They still used xylene to defat)
Please understand proper chemical handling & safety protocols for concentrated acids, strong bases and toxic organic solvents before performing this isolation.
Readers should also be aware that in some countries, including the US, the following is considered to be drug manufacturing and can result is serious legal trouble if put into actual practice.
The approach:
1) Prepare the material for extraction.
Starting amount for the following was 1 kilogram.
The bark was first broken into smallish pieces by hand and then run through an industrial blender in small amounts until it was all shredded and/or powdered.
2) Prepare an aqueous acid solution that is strong.
I have seen hydrochloric acid used as a 10% dilution (with more added if needed).
The process below used supersaturated citric acid.
This was prepared by heating 500 ml of water to boiling and adding 125 grams of citric acid with stirring. It was cooled before use.
3) Combine the acid with 3 Liters of Absolute ethanol denatured with isopropanol.
The resulting pH should be around 1.
Add more acid with stirring if needed.
pH2 works OK but the lower the better.
(Our belief is that this extreme acidic condition is degrading the tannins and preventing them from complexing with the alkaloids)
4) Soak material in the dark, at room temperature from overnight to a week.
5) Filter off the acidified alcohol and save it.
Be sure that no particulates came through your filter. Let stand and settle if they did.
(Repeat steps 2 & 3 another time or two but process them separately and carry the first extraction forward through to its completion as soon as is feasible)
6) Carefully reduce to a solid by evaporating the alcohol.
This will leave an acidic residue
7) Dissolve this residue in warm water
8) Defat this using xylene.
Perform the defatting a total of three times.
9) Basify to pH 14 using a very strong solution of lye (sodium hydroxide)
Lower pH will result in the aqueous phase forming two layers; a dark reddish one and a lower turbid blue-green-greyish one with much solids.
Also an abundant emulsion will form when the basic solution is shaken with a solvent.
Neither of these occurs at high pH.
If either one is an issue, just add more base.
10) Extract with toluene by carefully mixing for an extended period or by shaking.
Let stand until separated then draw off or pipette off the toluene.
11) Perform step10 a total of three times.
12) Evaporate the toluene.
If a rotovap is not available, a stream of air can be used to help but we would suggest using no heat.
The final stage of the evaporation should be in a largish flat bottomed glass dish.
13) When dry scrape up and package. Seeding should not be necessary.
This has reliably produced a yellow waxy-crystalline massive solid that crushed to white powder. It had only a faint floral smell indicating substantial purity and lack of skatole.
The reported recovery ran around 1% by weight using Brazilian-sourced Mimosa hostilis rootbark. (GC-MS of their result is beow).
Mexican material from Chiapas gave variable but significantly higher yields of 2-3%. So far no one we have encountered has acheived the 10% recovery reported by Holmstedt (in Ott).
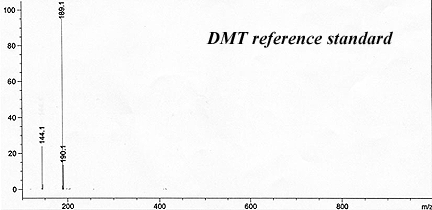
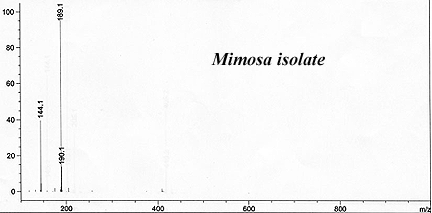
Callaway, James C. et al. (1999). "Pharmacokinetics of Hoasca alkaloids in healthy humans". Journal of Ethnopharmacology, 65: 243-256.
McKenna, Dennis J. et al. (1984). "Monoamine oxidase inhibitors in South American hallucinogenic plants Part 2: Constituents of orally-active myristicaceous hallucinogens". Journal of Ethnopharmacology, 12: 179-211.
Ott, Jonathan (1999). "Pharmahuasca: Human pharmacology of oral DMT plus harmine". Journal of Psychoactive Drugs, 31: 171-177.
Ott, Jonathan (2001). "Pharmepéna-psychonautics: Human intranasal, sublingual and oral pharmacology of 5-methoxy-N,N-dimethyl-tryptamine". Journal of Psychoactive Drugs, 33: 403-407.
Riba, Jordi et al. (2003). "Human pharmacology of Ayahuasca: Subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics". Journal of Pharmacology and Experimental Therapeutics, 306: 73-83.
There are no published analyses of vinho da jurema potions, but a 1983 collaboration between Karolinska Institutet and Universidade Federal do Paraiba in João Pessoa led to two independent analyses of jurema preta potions obtained from an Umbandista juremeira in Alhandra, Paraíba. The first, dated 12 November 1983, was analyzed in Sweden and reported to contain 1-10 mg/mI DMT; while a more precise quantitative analysis in João Pessoa of a potion said to be "identical to the sample taken to Karolinska" but dated 5 December 1983, found 7.46 mg/ml DMT, with the source root-bark containing 11% DMT (HOLMSTEDT 1983; SANCHEZ LEMUS 1984)! The Brasilian group found DMT also in a jurema branca sample from Alhandra, unfortunately unidentified.
Thus we have a range of reported DMT concentration in jurema preta root-bark from 1-11% [since PACHTER's group isolated 0.57% DMT, we can assume a total content perhaps twice as high]; as compared to 0.00-0.66% DMT in reported analyses [12 samples] of Psychotria viridis or chacruna leaf, chief source of tryptamines for ayahuasca potions. Even taking the highest DMT level found in chacruna, jurema preta is 1.5-16.5 times richer in DMT! As for the potions, we have no data on amounts of vinho da jurema typically consumed, but 7.46 mg/ml DMT would correspond to 0.45-1.64 g DMT per dose, taking the range of 60-220 ml reported for typical doses of ayahuasca, analysis of which found 25 mg[220 ml]-36 mg[60 ml] DMT/dose, meaning that VINHO DA JUREMA may be 12.5-65 times higher in DMT than ayahuasca (OTT 1994, 1999)! The vinho da jurema potions analyzed were said to be thick residues or concentrates; even allowing for a 10-fold concentration prior to analysis, vinho da jurema remains 1.25-6.5 times higher in DMT than ayahuasca. Inasmuch as the 36 mg[60 ml] DMT in ayahuasca doses was from Pucallpa and Tarapoto, Perú, where the brews are also considerably concentrated, we can assume the vinho da jurema analyzed to be at least 2.5-3.0 times higher in DMT than typical ayahuasca.
5-MD (Ott)
You didn't miss it; I don't have it. I don't have the first issue and the last issue in my collection. The first issue is OOP, but I think that the last issue (which has the Ott article) is still available from the publisher. It might be here:
http://www.vwb-verlag.com/Katalog/m033.html

