Methadone (1)1 was first synthesized as the racemic modification by Bockmuhl and Erhardt2 according to the approach outlined in Scheme 1. Treatment of diphenylacetonitrile anion (2) (as the lithium, sodium or potassium salt) with either 1-dimethylamino-2-chloropropane (3) or 2-dimethylamino-1-chloropropane (4) produced a mixture of isomeric nitriles 5 and 6. This reaction is extensively documented in the literature2-19. The aziridinium ion intermediate (7) has been proposed to account for the production of rearranged products (5 and 6) and for the stereochemical course17 in the key alkylation step. Reaction of 5 with ethyl magnesium bromide followed by acid hydrolysis afforded methadone (1) in high yield.
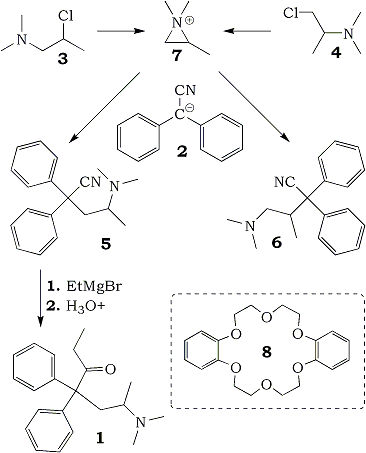
Under Bockmuhl’s original conditions (sodamide in benzene as base)2, the alkylation proceeds without selectivity and a nearly 50:50 ratio20 of nitriles 5 and 6 is observed. In fact, nucleophilic attack of 2 on the less hindered carbon of 7 is expected to occur with a lower activation energy than attack on the methyl-substituted carbon.
Accordingly, in an effort to produce 5 in higher proportions, modified reaction conditions were explored aiming principally at varying solvents, bases and reaction temperatures.
The results are given in Table 1. There appears to be a significant correlation between lower reaction temperatures and higher proportions of 5 over 6. However, when the reaction was run at RT even for a long period of time, (2.5-4.0 days), a low conversion of diphenylacetonitrile was encountered (12-15%). To circumvent this drawback (by enhancing the reactivity21,22 of 2, dibenzo-18-crown-6 (8) was used under both liquid-liquid and liquid-solid phase PT conditions.
It is a well-established fact that crown ethers form stable complexes with metal cations23, and by increasing the dissociation of ion pairs they provide highly reactive, unsolvated (so-called “naked”) anion species24. However, in homogeneous conditions, their practical importance is limited, as they must be employed in equimolar amounts. On the contrary, in two-phase organic aqueous systems, crown ethers of suitable structure (dibenzo-18-crown-6 (8) or dicyclohexyl-18-crown-6) are effective in catalytic amounts and act as PTC’s. Best results in the production of 5 were obtained under liquid-liquid two-phase conditions in the presence of 8.
Optimization of reaction conditions led to a striking 72:28 ratio of 5 to 6 ensuring an 86% overall yield of (5+6) and 44% of recrystallized 526. This compares most favorably with the various homogeneous systems presented in Table 1 (#3-7, 10), and particularly with the 26% of isolated 626 in Bockmuhl’s original work2,20.
Solid-liquid two-phase systems in conjunction with crown ethers offer a valuable alternative to liquid-liquid two-phase conditions. Crown ethers and sodium or potassium carbonate have been used successfully to effect alkylation of carbanions from various substrates, including nitriles possessing acidic C-H bonds29.
Exp # |
Solvent | Temp (°C) Time (h) |
Base | Relative Proportion |
Yield (5+6) |
Yield of 5e |
|
5 |
6 |
||||||
1 | Xylenea,b | 135/2.5 | KOH | 58 | 42 |
98% |
38% |
2 | Xylenea,b | 135/2.5 | K2CO3 | 58 | 42 |
10% | - |
3 | Toluenec | 110/2.5 | NaH | 54 | 46 |
45% |
13% |
4 | Dioxanec | 100/2.5 | NaH | 60 | 40 |
83% |
32% |
5 | Benzene2,17 | 80/2.5 | NaNH2 | 48 | 52 |
83% |
26% |
6 | THFc | 65/2.5 | NaH | 65 | 35 |
83% |
36% |
7 | THF/t-BuOHc | 65/2.5 | t-BuONa | 63 | 37 |
85% |
36% |
8 | DMSO/H2Oa,d | 45/2.0 | NaOH | 72 | 28 |
85% |
44% |
9 | DMSO/H2Oa,d | 20/48 | NaOH | 75 | 25 |
65% |
34% |
10 | THFc | 20/96 | NaH | 70 | 30 |
15% | - |
11 | DMSO/H2Of,d | 45/1.0 | NaOH | 69 | 31 |
75% |
39% |
Notes
- These experiments were performed in the presence of 8.
- These experiments were conducted as in C.
- These experiments were run as in A.
- Experimental conditions can be found in B.
- After r ecrystallization from n-heptane, except for #526
Yields are calculated on the basis of diphenylacetonitrile. - Benzyltriethylammonium chloride (5 g) used instead of 8.
Nevertheless, this solid-solid system suffers two main disadvantages: high temperatures (120-150°C) and low yields (35-45%). In the present case, the required high temperatures were expected to produce low selectivity and this was indeed the case (see Table 1, #2). More effective conditions were found when solid KOH was used. (#1).
Our results (#1 and #2) parallel the data recently reported by Dou et al concerning the use of solid K2CO3 and solid NaOH in the Williamson ether synthesis: the superiority of solid NaOH over K2CO3 was clearly demonstrated30.
Two-phase liquid-liquid systems, such as the one described here, offer numerous advantages over homogeneous reaction conditions using NaH, NaNH2, or t-BuOK : hazardous and expensive reagents, anhydrous solvents, nitrogen atmosphere… are rendered unnecessary and the actual procedure can be of practical interest in the preparation of radio-labeled methadone or stable-isotope analogues of methadone, as this modification of Bockmuhl’s synthesis makes the best use of costly precursors.
Experimental
(R,S)-2,2-Diphenyl-4-dimethylaminopentanenitrile (5)
A. Homogeneous Conditions
Into a dry, three-necked flask (500 ml) fitted with dry N2 inlet, septum and reflux condensor equipped with a CaCl2 tube were placed 150ml of dry THF and 10.5g of a 50% suspension of NaH (0.22 mol) in mineral oil. A solution of 42.5g of diphenylacetonitrile (0.22 mol) in 100ml of dry THF was added dropwise over 1 hr after which time a deep red color had developed. A solution of 30.4g of 3 (0.25 mol) in 60ml of dry xylene was then added in one portion. The resulting reaction mixture was stirred and refluxed for 2.5 hr. After cooling to RT, the suspension was poured into 1L of ice-water and extracted three times with Et2O. The dried (MgSO4) extracts were evaporated under-reduced pressure to remove most of the THF and then taken up in Et2O. The product was extracted into 10% aq. HCl; the aqueous extract was back-washed with Et2O and made basic with 10% NaOH. The resulting oil was taken up in Et2O and dried over MgSO4. The solvent was removed in vacuo to give an oily residue (51.6g, 83% yield) consisting essentially of a mixture of 5 and 6. This oil was chilled and triturated in cold n-hexane (400ml) to produce a white product, that was collected by filtration and recrystallized from n-heptane (400ml) to yield after storage in the refrigerator 22g of 5 (36% yield) that had; MP: 90-91°C6. 1H-NMR, IR and MS were consistent with the proposed structure. This compound was pure by GLC and HPLC.
B. Liquid-Liquid Two-phase Conditions
To a solution of 60g of diphenylacetonitrile (0.31mol) and 2g of 8 (5.5 mmol) in 50ml of DMSO was added under efficient mechanical stirring a solution of 50g of NaOH (1.25 mol) in 50ml of distilled water; 60g of the HCl salt of 3 (0.38mol) was then added in small portions over 1 hr. This resulted in a stepwise increase of the temperature up to 45°C. The two-phase system was stirred during and additional hour while the temperature was kept at 45-50°C with a water bath. The work-up procedure was performed essentially in the same way as in A to give 74.2g of the mixture of 5 and 6 (86% overall yield). Trituration in n-hexane, filtration and recrystallization from n-heptane yielded 38g of 5 (44% yield). MP: 90-91°C. This compound was pure by HPLC and GLC.
C. Solid-Liquid Two-phase Conditions
To a solution of 10g of diphenylacetonitrile (51.7 mmol) in 50ml of dry xylene were added 1 g of 8 (2.75 mmol) and 15g of solid KOH in granules (0.263 mol). The mix was heated to reflux and a solution of 10g of 3 (82.3 mmol) in 25 ml of dry xylene was added portionwise over 15 min. After refluxing for 2.5 hr, the supernatant reddish liquid was decanted from the solid residue and filtered. The usual work-up afforded 5.5g (38% yield) of recrystallized 5; MP: 90-91°C.
GLC Conditions of analysis of 5 and 6.
Glass column 3% OV 17 (2 m, i.d. 3 mm) Flow rate 30ml/min. Oven temp. program; start 140°C, 8°C/min increase to 200°C. Compound 6 had Rt 11.1min; compound 5 had Rt 12.8 min.
HPLC Conditions of analysis of 5 and 6.
Partisil 10 PAC, length 30cm, int. diam. 4.6mm, flow rate 1.3mol/min solvent: n-hexane, methanol, n-butanol, 60:20:20 (v/v). Compound 6 had K’ 2.27 and compound 5 had k’ 3.51. Ethanol was considered as the unretained compound.