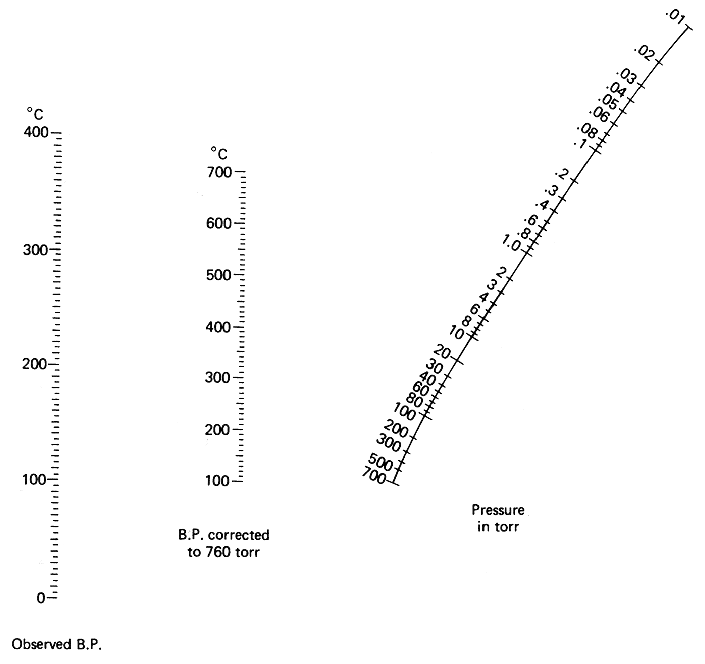
The picture below is a so called Nomograph. With the aid of it and a ruler, you can determine at what temperature a certain liquid will boil under vacuum. You can also use it the other way around to determine the strength of your vacuum.
Begin with printing the picture, as it will then be much easier to use the Nomograph.
Determining the boiling point of a liquid under a certain vacuum
In this example, we assume that your vacuum source pulls 20 mmHg (20 torr), and that you want to determine the boiling point of water at that vacuum. The boiling point of water at normal pressure is 100°C. With the aid of a ruler, draw a line from 20 mmHg in the pressure graph (to the right), through 100°C in the middle graph ("boiling point corrected to 760 torr", this is the normal atmospherical pressure), and where this line intersects the line to the left ("Observed boiling point"), take your reading. About 15°C, right? This means that at a vacuum of 20 mmHg, water will boil already at 15°C, below normal room temperature.
Determining the strength of an unknown vacuum
You have bought a brand new vacuum pump, but you don't know how much it will pull. The nomograph can help you with this too. To do this, you need to distill a liquid with a known boiling point in your distillation apparatus with your vacuum pump attached. We will use water in our example again. When distilling, you observe that the water comes over at a temperature of 40°C after you have waited for the temperature to stabilize. You know that the normal boiling point of water is 100°C. Draw a line through 40°C in the leftmost graph, through 100°C in the middle one, and notice where the line intersects the pressure graph. About 100 mmHg, right? This is how much your new vacuum pump will pull.
Note: All readings are approximate. The exact data depends on the nature of the distilled compound, and may differ by ten percent or so.