These german patents from the 1930's all describe the rearrangement of morphine, codeine and ethylmorphine into the corresponding dihydromorphinones. The first uses immense amounts of platinum or palladium in acid solution to affect the rearrangement, the second uses a lot less catalyst, and the last one uses alcohol as solvent, in which the reaction proceeds without any acid added.
Procedure for the Production of Dihydromorphinones1
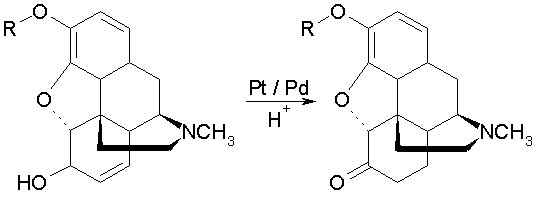
It is well known that morphine and its ethers, through treatment with hydrogen gas in the presence of large amounts of noble metal catalysts through heating the acid solution, can be transformed to the dihydrated keto derivatives.
Surprisingly, it was now found that for this transformation to take place, hydrogen is completely unnecessary, and that just heating the acidified alkaloid solution with a larger amount of finely divided platinum row metals, will suffice to affect the simultaneous dehydrogenation/hydrogenation in good yields.
This success is particularly unexpected because it was not to be foreseen that platinum metal not saturated with hydrogen is capable to use the hydrogen originating from the dehydrogenation [of the alcohol in the 6-position] almost quantitatively for the hydrogenation of the double bond [in the 7-8 position] of morphine or its analogs without in addition of excess hydrogen from an external source.
Example 1:
300 grams of codeine free base was dissolved in 2000ml dilute HCl, and after the addition of 150 grams finely divided palladium, the mixture was heated under reflux for one hour. After the palladium was filtered off, the acid filtrate was basified with NaOH solution. The base which separated was recrystallized from alcohol. Yield of dihydrocodeinone 75-85%, mp 195°C.
Example 2:
5 grams of codeine hydrochloride was dissolved in 30 ml water, and acidified with a little HCl, and after the addition of 4 grams platina black, the solution was refluxed for 5 hours. After workup as in Example 1, the yield was 65-75% of theory.
Example 3:
5 g of morphine HCl was dissolved in 50ml water, slightly acidified with HCl, and 5 grams of platina black was added and the solution refluxed for 4 hours. Workup as in Example 1. Yield 2.5 grams dihydromorpinone.
Example 4:
To a solution of codeine hydrochloride, slightly acidified with HCl, 50 ml of a 10% solution of colloidal platinum was added, and the solution was refluxed for 3 hours. Workup as in example 1. Yield 3 g dihydrocodeinone.
Example 5:
6 grams codeine freebase was dissolved in a solution of 4 grams of tartaric acid in 50 ml of water, and 6 grams palladium black was added. The solution was refluxed for 1 hour and worked up as in Example 1. Yield 75% of theory.
Example 6:
6 g codeine phosphate was dissolved in 30 ml water, and acidified with 5ml dilute phosphoric acid. 4g of finely divided palladium was added, and the solution was refluxed for one hour. Workup as in Example 1. Yield 60-70%.
Example 7:
6 grams of codeine free base was dissolved in dilute acetic acid, and was refluxed for two hours after the addition of 5 grams palladium black. After workup as in Example 1, the yield was 40% of theory.
Example 8:
21 grams of codeine free base was dissolved in an excess of dilute sulfuric acid, mixed with 16 grams palladium black and refluxed for one hour. After workup as in Example 1, the yield was 80-85% of theory.
Example 9:
10 grams of ethylmorphine was mixed with 50 ml water and acidified with HCl. 10 grams of platinum black was added and the mixture was refluxed. The yield of ethyldihydromorphinone was 65-75% of theory.
Procedure for the Production of Dihydromorphinones2
As in German Patent 607,931, a method is decribed below for the production of dihydromorphinones by rearrangement without the use of hydrogen, but simply by heating the alkaloid in an acidic solution together with a platinum catalyst.
While relatively large amounts of platinum catalyst is used in German Patent 607,931, we have found that only a fraction of that amount is needed, and sometimes with even better yields.
Example 1:
300g codeine freebase was dissolved in 2000ml dilute HCl, and was heated with 25g of finely powdered palladium, and boiled under reflux for an hour. The palladium was filtered off, and the filtrate basified with NaOH solution. The precipitated base was recrystallized from alcohol. Yield 85-95% of dihydrocodeinone, mp 195°C.
Example 2:
5 grams of codeine hydrochloride was dissolved in 30ml water, and acifified with a small amount of HCl, and after the addition of 0.5g of palladium black, the mixture was refluxed for five hours, and worked up as in example 1. The yield was 70% of theoretical.
Example 3:
5 grams of morphine hydrochloride was dissolved in a slightly acid HCl solution, and after the addition of 0.5g palladium black, the mixture was refluxed for three hours and worked up as in Example 1. The yield was 3g.
Example 4:
6 grams of codeine freebase was dissolved in 30ml water together with 4g of tartaric acid, and 0.5g of palladium black was added. The mixture was refluxed for one hour and worked up as in Example 1. The yield was 75% of theory.
Example 5:
6 grams of codeine phosphate was dissolved in 30ml water, and acidified with 5ml dilute phosphoric acid, and after the addition of 0.5g of palladium black, the solution was refluxed for an hour. After workup as in Example 1, 60-70% yield of dihydrocodeinone was isolated.
Example 6:
21 grams of codeine freebase was dissolved in an excess of dilute sulfuric acid, and two grams of palladium black was added and the solution refluxed for one hour. The yield was 90% of theory.
Example 7:
10 grams of ethylmorphine freebase was added to 50ml water, and HCl was added until the solution was slightly acidic and all ethylmorphine had dissolved. 1 gram palladium black was added, and the solution was boiled under reflux. The mixture was worked up as in Example 1. The yield of ethyldihydromorphinone was 75% of theory.
Procedure for the Production of Dihydromorphinones3
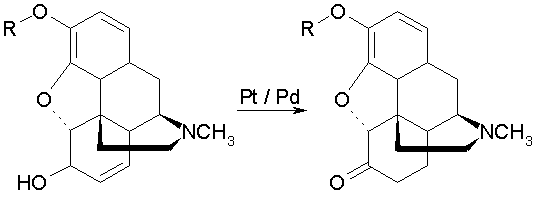
As in German Patent 617,238, a method is decribed below for the production of dihydromorphinones without the addition of hydrogen gas.
While the rearrangement in German Patent 617,238 is affected by the presence of acids, we have now found that the reaction with morphine and its ethers is taking place in an alcoholic solution in good yields without the addition of any acid.
Example 1:
5 grams morphine was dissolved in 50 ml of alcohol and after the addition of 0.5g of palladium black, the mixture was refluxed for 4 hours. After the catalyst wass filtered off and the solution concentrated, dihydromorphinone crystallized. Yield 3 grams.
Example 2:
10 grams of codeine was dissolved in 100ml alcohol, and after the addition of 0.5 grams palladium black the solution was refluxed for 4 hours. Workup as in Example 1. Yield 7 grams of dihydrocodeinone.
Example 3:
5 g ethylmorphine and 0.3g of palladium black was dissolved in alcohol and the solution was refluxed. Yield 3g of ethyldihydromorphinone.