Abstract
Historically, drugs of abuse have come from two sources: plant products and diverted pharmaceuticals. Today, new, totally synthetic drugs produced by clandestine laboratories have become an increasingly important source of abused substances. Of particular concern are the fentanyls, a family of very potent narcotic analgesics, which first appeared on the streets in California in 1979 under the name "China White." At least 10 different analogs have been identified to date and are thought to be responsible for over 100 overdose deaths. The fentanyls are not used by any particular ethic or age group, but rather by the general heroin using population. Their use, however, does seem to be restricted to suburban, rather than urban areas, and almost exclusively to the state of California. The most potent analogs, the 3-methyl- and beta-hydroxy-fentanyls, may be up to 1000 times as potent as heroin, but are not chemically related to the opiates and therefore not detected by conventional narcotic screening tests. However, using a sensitive radioimmunoassay highly specific for the fentanyls they can be measured at the very low concentrations observed in body fluids, generally less than 10 ng/mL. It is likely that, as efforts to restrict the importation of natural products and prevent diversion of pharmaceuticals become more effective, the fentanyls and other synthetics will become increasingly important drugs of abuse.
"Designer drugs" is a term that has been used recently to describe synthetic drugs of abuse1,2. It is not a precise scientific term and has been indiscriminately applied to a variety of contemporary drugs of abuse. More correctly, this term should be applied to only those drugs that are (1) synthesized from common chemicals, (2) exempt from control by the Drug Enforcement Administration because of their unique chemical structure, and (3) skillfully marketed under attractive, often exotic names.
Past History
The use of drugs for medical and non-medical purposes can be traced back to man's early history for as Aldous Huxley said, "Pharmacology is older than agriculture." Until the nineteenth century, drugs came from two sources - unrefined plants and animal products - and were usually taken by only one route of administration - oral ingestion. Eating crude plant material offered a certain safety margin since biologically active components are usually present in small amounts and overdosing was physically difficult.
It might be argued that a major trend throughout the history of drug use and abuse is the increased hazards associated with the use of more potent drugs, either purified plant materials or new synthetic compounds. Drugs became more potent as chemists were able to extract and purify the active ingredients present in botanicals. Around 1000 A.D., distillation techniques were applied to fermented beverages. This resulted in a product that was ten-fold more potent, which could be flavored, and thus was more pleasant to consume. In the mid-1800s morphine was isolated from opium; in the early 1900s, cocaine was isolated from coca leaf and heroin was synthesized from morphine. The abuse of these purified, more portent materials soon followed.
The next significant change in the patterns of drug abuse came with the introduction of pharmaceuticals synthesized from organic chemicals. As drugs with psychoactive properties were developed (for example, amphetamines and barbiturates) and introduced into clinical use, their diversion and abuse became a problem. When abuse of these compounds reached unacceptable levels, their production, distribution, and use were more tightly controlled. Clandestine laboratories soon appeared to meet the black market demand. Throughout the 1960s and 1970s as state and federal governments became more successful in curtailing the importation of natural products and the diversion of pharmaceuticals, the number of illicit laboratories producing contraband drugs increased.
One of the earliest examples of this phenomenon is lysergic acid diethylamide (LSD), It is estimated that over 250 000 doses of LSD were distributed for research purposes by the Sandoz Pharmaceutical Company between 1955 and 1965. When adverse publicity over the recreational use of LSD prompted Sandoz to restrict severely the supply of LSD, the first clandestine LSD laboratories surfaced to meet the demand. In 1967, shortly after Parke-Davis withdrew phencyclidine as a pharmaceutical, the first PCP laboratories appeared. Similarly, when the Comprehensive Drug Abuse Prevention and Control Act of 1970 put production quotas on amphetamines and barbiturates, clandestine amphetamine laboratories appeared.
Beginning in 1979, the illicit synthesis of drugs was elevated to an extraordinarily sophisticated level. In the last week of December in 1979 two very unusual overdose deaths occurred in Orange County, California. Both victims had a history of heroin use, were found with paraphernalia, and the autopsy findings of pulmonary edema and "tracks" were consistent with a heroin overdose. Surprisingly, toxicological analysis of the body fluids showed no drugs present. Over the next few months six more overdose deaths occurred in Orange County, and by the end of 1980 there was a total of fifteen deaths, all of which appeared to be classic narcotism deaths, yet no drug could be found. During this same period, law enforcement officials seized a number of street samples which were being sold as heroin, but which contained no heroin or, in fact, any other drug. A common link between these events was that the overdose victims were buying and the drug the dealers were setting a drug called "China White" or occasionally "synthetic heroin"3.
Following a series of investigations, the Drug Enforcement Administration reported the material being sold as "China White" was a drug called 3- methylfentanyl4. This identification proved to be incorrect and later the drug was correctly identified as alpha-methylfentanyl. Alpha- methylfentanyl is a very simple analog of the pharmaceutical fentanyl, a very potent narcotic analgesic synthesized in the early 1960s by the Janssen Pharmaceutica research laboratories in Belgium5. Now, for the first time, illicit laboratories were producing original drugs. This new drug was not an illicit copy of a pharmaceutical, and therefore, had not been subjected to toxicological studies or clinical evaluation in human subjects. Further, even though alpha- methylfentanyl was chemically and pharmacologically nearly identical to fentanyl (alpha-methylfentanyl is twice as potent as fentanyl), it was technically a new drug entity, not on the DEA's list of restricted drugs, and therefore a legal drug. In fact, alpha-methylfentanyl remained a legal drug until 1982 when it was classified a Schedule I drug6.
The fentanyls are a large family of highly potent synthetic narcotic analgesics. They hate all the properties of the opiates and opioids, yet are chemically unrelated, and therefore do not cross-react with any of the reagents used in opiate screening tests. The parent drug fentanyl was introduced into clinical medicine in Europe in the 1960s and in the United States in the early 1970s under the tradename Sublimaze®. It is widely used clinically, sometimes in combination with the major tranquilizer droperidol (Innovar®), as a preanesthetic medication, an anesthetic, and a postsurgical analgesic7. Fentanyl's use in veterinary medicine is limited to surgery in dogs because, like other narcotics, it produces behavioral excitation in some species such as the horse, cat, and mouse, Fentanyl is approximately 200 times as potent as morphine, has a very prompt onset of action (1 to 2 min) and a short duration of action (approximately 40 min)8,9. Like other narcotic analgesics, respiratory depression is the most significant acute toxic effect of the fentanyls. The depth and duration of respiratory depression depends on the analog used and the dose administered. As with other narcotics, naloxone is the antidote of choice for respiratory depression.
Our laboratory became active in the investigation of these new unexplained overdose deaths because we had developed a very sensitive and very specific radioimmunoassay for fentanyl shortly after its introduction into clinical medicine10. Our laboratory first employed the assay in pharmacokinetic studies in surgical patients11. Later, when it came to our attention that fentanyl was being used to "dope" race horses12, we applied our method to the detection of fentanyl in body fluids of race horses and were able to isolate and identify the primary metabolite of fentanyl which is excreted in urine and which is the antigen detected by radioimmunoassay (RIA)13.
When we examined the body fluids of the "China White" overdose victims using our RIA technique, we identified a fentanyl-like compound and confirmed its structure as alpha-methylfentanyl. The drug was present in blood and urine in very small concentrations, between 1- and 10-ng equivalents of fentanyl per millilitre, and was present in powder samples generally at about 1 µg/g14.
Alpha-methylfentanyl was placed on the restricted drug list as a Schedule I drug on 22 Sept. 1981, but by this time another analog of fentanyl had appeared - para- fluorofentanyl. This new compound had approximately the same potency as fentanyl, but appeared on the street only briefly.
In the spring of 1984, yet another analog was introduced - alpha-methyl acetylfentanyl. Although this new analog differed from alpha-methylfentanyl by only one methyl group, it was technically a new chemical entity, not a restricted drug, and therefore completely legal. This compound was always found contaminated with the acryl analog, most likely a by-product formed during synthesis. Alpha-methyl acetylfentanyl is less potent than fentanyl (about ten times as potent as morphine), but longer acting than fentanyl (just slightly shorter acting than morphine).
The succession of new fentanyl analogs appearing on the streets continued. In March of 1984 we identified 3-methylfentanyl in powder samples found near a suspected drug overdose victim. The appearance of this particular fentanyl analog was yet another surprise, for although the structure of 3-methylfentanyl was remarkably similar to previous analogs, it was much more potent. In fact, 3-methylfentanyl is probably the most potent analog of the fentanyl series and one of the most potent narcotic analgesics known. According to Janssen Pharmaceutica, who had reported its synthesis earlier, the cis isomer of 3-methylfentanyl is approximately 6000 times as potent as morphine while the trans isomer is approximately 400 times as potent15.
At this time, the number of fentanyl overdose cases sent to our laboratory increased dramatically. During 1983 we were identifying approximately one fentanyl case every two months; during 1984 this number quickly rose to one case each week. Concentrations of this new analog in body fluids were at or below the 1-ng level, much lower than for the previous analogs. Also, we observed that all samples contained both the cis and trans isomers of 3-methylfentanyl and fentanyl as well. The small amounts of fentanyl present probably result from the incomplete methylation of the piperidine ring, 3-methylfentanyl disappeared almost as quickly as it appeared; it was available in California from the Spring of 1984 until the Spring of 1985.
Once again, there was another surprising turn of events with the virtual disappearance of fentanyls from the streets. Both the incidence of fentanyl overdose deaths and the number of street samples containing fentanyl decreased sharply. Even more surprising, the 3-methyl analog was replaced by a collection of even more exotic analogs. The last powder samples of "China White" we obtained in 1985 contained not 3-methylfentanyl, but a complex mixture of beta-hydroxy-, alpha-methyl-, and 3-methyl- substituted fentanyls and thienylfentanyls.
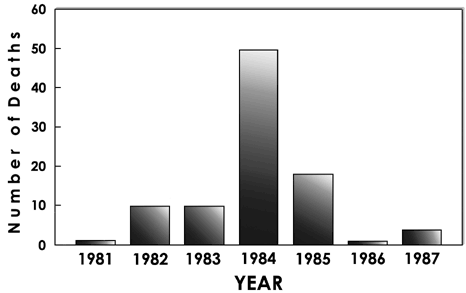
Fig. 2
Fentanyl overdose deaths identified by our lab at UC Davis, CA.
Curiously, we have seen fentanyl itself sold as "China White," most often in the San Diego area. This material must be of clandestine origin, not diverted pharmaceutical stocks, because it is always contaminated with benzylfentanyl. Benzylfentanyl is not pharmacologically active as a narcotic, but is a logical precursor in the synthesis of fentanyl.
The physical appearance of seized powder samples of the fentanyls examined by our laboratory is as diverse as the names under which they are sold. The various fentanyl analogs are diluted (cut) with large amounts of lactose or mannitol so the amount of active drug present is exceedingly small, generally less than 1%, and therefore contributes nothing to the color, odor, or taste of the sample. The color of the samples ranges from pure white (which might be sold as "Persian White"), to an off-white or light tan (sold as "China White," "synthetic heroin," or "fentanyl"), to light or dark brown (sold as "Mexican Brown"). The brown color apparently comes from lactose which has been heated and slightly caramelized. The texture of the samples ranges from light and finely powdered to somewhat coarse, cake-like, and crumbly, resembling powdered milk. Occasional samples will have a medicinal or chemical odor, but this is not characteristic. The fentanyls, therefore, can appear in all the various forms that heroin does and there is nothing about the appearance of any sample that will identify it as containing fentanyl.
Present Status
To date, we have identified over 10 illicit fentanyl analogs and 110 fentanyl overdose deaths. All deaths occurred in California with the exception of 2 cases in Portland, Oregon (1983), 1 case in Tempe, Arizona (1980), and 1 case in Reno, Nevada (1987). A preliminary review of the investigation reports and coroners findings show that the fentanyl overdose victims typically were young (mean age 30, range 18 to 49), male (74% male, 26% female), employed as a blue-collar worker, and found at home (usually in the bathroom or bedroom) by family or friends. Most often they had a prior history of heroin use, but often there were claims by the family and friends that the individual had not been abusing drugs recently. Generally, no other drugs were found; however, if another drug was present, it was invariably ethanol, most often present at the 0.1% level. Thus the profile of the typical fentanyl overdose victim suggests that the drugs are not distributed within any localized geographical area or to any particular ethnic or age group. Instead, the fentanyls seem to be distributed rather uniformly throughout the addict population. However, the geographic distribution of the fentanyl deaths is somewhat curious. Most deaths occurred in suburban, even rural areas, rather than large cities. There has never been a illicit fentanyl death in the city of Los Angeles and only 2 deaths in San Francisco. This unusual distribution remains unexplained but is not the result of underreporting because our laboratory routinely examines body fluids submitted by coroners in these two cities.
Currently, the only illicit fentanyl available is the fentanyl/benzylfentanyl mixture and is confined primarily to the San Diego area. During the first six months of 1987 we identified four fentanyl related deaths. Three of the overdose cases occurred in California and one in Nevada. In two of these cases, the fentanyl and benzylfentanyl mixture was identified in the body fluids and accompanying paraphernalia. In the other two cases, the victims were health professionals anti their deaths appeared to be the result of abuse of pharmaceutical fentanyl.
Future Trends
In the view of this author, it is likely that the future drugs of abuse will be synthetics rather than plant products. They will be synthesized from readily available chemicals, may be derivatives of pharmaceuticals, will be very potent, and often very selective in their action. In addition, they will be marketed very cleverly.
The "Designer Drug" problem may become an international problem. A single gram of any very potent drug like 3-methylfentanyl could be synthesized at one location, transported to distribution sites worldwide, and then formulated (cut) into many thousand, perhaps a million, doses. Preventing the distribution of such small amounts of the pure drug will be exceedingly difficult.
Fortunately, wide distribution of the fentanyls has not yet occurred. Use of the illicit fentanyls has been restricted almost exclusively to California, and at the present time, they do not appear to be widely available, even in California. However, there were significant events in 1986 which suggest that the fentanyls could reappear at any time and would probably not be restricted-to California. During 1985 and 1986, at least two, and possibly four, well-trained organic synthetic chemists were arrested for synthesizing 3-methylfentanyl16-18. Even more disturbing is the fact that they were working in legitimate chemistry laboratories on the East Coast, not in California. Fortunately, these chemists were apprehended before they were able to distribute any of the drug. Again, it is important to remember that any chemist who is successful in achieving even a nominal yield of a few hundred milligrams of any of the potent fentanyls has at his disposal many millions of individual doses of a synthetic heroin substitute.
What about other drugs classes? The literature abounds with synthetic routes and pharmacological properties of thousands of narcotics, stimulants, hallucinogens, and sedative-hypnotic drugs. This information is so readily accessible that creative clandestine chemists can continue to exploit the pharmaceutical chemistry literature. Restricting access to the chemical literature is not feasible and controlling the chemicals needed to make these drugs may be only minimally effective. In the past, restricting certain chemicals has only stimulated clandestine chemists to assemble the drugs from more elementary precursors. Locating these laboratories will also be a difficult task. When very potent chemicals are produced, a clandestine laboratory need operate for only a short time to make a few hundred grams of material. This problem is compounded by the recent trend of chemists synthesizing illicit drugs in legitimate chemical laboratories. Our traditional responses to drug abuse problems seem to offer little promise. In fact, any success we may have in curtailing the distribution of natural products such as opium, coca, and marijuana and preventing the diversion of pharmaceuticals will only stimulate the development of potent synthetic substitutes.
The challenge for the forensic chemist is to keep pace technologically. The increased use of very potent, new drugs of abuse will require new screening techniques capable of detecting a wider variety of chemical structures, as well as more sensitive methods that can measure these drugs and their metabolites at the nanogram and picogram level.
Simple answers to this growing problem are not readily apparent, but we are not likely to find them unless the problem is first recognized.