Abstract
Oxidation of Beta-asarone (2) with DDQ gave trans-2,4,5-trimethoxycinnamaldehyde (3), which on treatment with p-toluenesulfonyl hydrazine provided corresponding alpha,beta-unsaturated hydrazone derivative (4). Reduction of 4 with sodium borohydride in acetic acid afforded gamma-asarone (1) in 43% yield.
In nature asarones1 exist in three isomeric forms, namely, alpha-,beta, and gamma-asarone (trans-2,4,5-trimethoxy-1-propenylbenzene, cis-2,4,5-trimethoxy-1-propenylbenzene, and 1-allyl-2,4,5-trimethoxybenzene, respectively). gamma-Asarone (1) is a rare phenylpropanoid2 first isolated from Caesulia axillaries3and later detected as a biologically active4 constituent of various essential oil bearing plants5.
However, no simple method is available for separation and isolation of asarones (alpha, beta and gamma) in single isomeric form due to their similar physical properties. Separation of less abundant 1 via column chromatography of asarones-rich essential oil is particularly difficult. A few methods for the synthesis of 1 are found in the literature,2,6 but protocols either involve multiple steps starting from dimethoxyphenol6a,c with overall poor yield or require careful handling of sodium perchlorate during electrolysis of methyl eugenol.2,6b
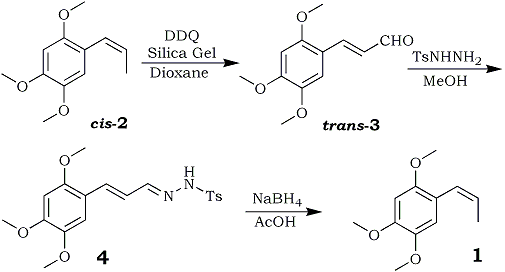
Herein we report a simple synthesis of 1 from toxic beta-asarone7 (2) via formation of a key intermediate, 2,4,5-trimethoxy- cinnamaldehyde (3), as outlined in Scheme 1; compound 3 is itself a rare phenylpropanoid found in traces in C. axillaries8 and Alpinia flabella9.
Beta-Asarone (2) is found in several plants1 including Acorus calamus10 (family Araceae). The high percentage of toxic 2 (varying from 70 to 90% in tetraploid and hexaploid strains11 distributed extensively in India, Pakistan, Bangladesh, Japan, and China) restricts the market potential12 of calamus oil. Therefore, as a part of our continuing efforts to generate value-added products,13 compound 2 has been converted into rarer phenylpropanoids 3 and 1. Treatment of 2 (cis-isomer) with DDQ14 in wet dioxane afforded 3 in 41% yield, whereas the addition of a catalytic amount of silica gel (60-120 mesh size) improved the yield of 3 up to 62%. 1H NMR clearly indicated the formation of 3 as trans-alpha,beta-unsaturated aldehyde in which the olefinic proton appeared at 7.81 (J=15.8 Hz) rather than the expected cis-isomer.15 Alternatively, the above oxidation was repeated starting with trans-asarone, which produced an 84% yield of expected trans-cinnamaldehyde (3), identified by mixed melting point and comparison of its spectral data with an authentic sample8,13c.
These results clearly indicated formation of the thermodynamically more stable trans isomeric form of cinnamaldehyde (3) whether starting from cis- or trans-asarone; however, trans-asarone provided higher yields. Compound 3 has been isolated, so far, from two plants,8,9 but only in trace amounts. Treatment of a methanolic solution of 3 with p-toluenesulfonylhydrazine16 (1.2 equiv) afforded the corresponding alpha, beta-unsaturated tosylhydrazone derivative (4) in 79% yield. To obtain the product 1, the reduction and double-bond migration17 of tosylhydrazone derivative 4 proceeded smoothly and in good yield (43%) with acetic acid-sodium borohydride, but yields were lower using sodium cyanoborohydride-sulfolane. The Rf value (0.39 in 4% ethyl acetate in hexane) of 1 was exactly the same as that for 2; however, spectral data of 1 were found consistent with those in the literature.1,6c Finally, we conclude that the synthesis of 3 and 1 from 2 is convenient and efficient in comparison to reported2,6,8 methods.
Experimental
Plant Material and Isolation of Beta-Asarone (2).
The rhizomes of Acorus calamus were collected from Palampur (1300 m altitude) in May-June 1997, and the plant specimen was compared against a voucher specimen (no.1066) in the herbarium of the IHBT, Palampur, India. The steam distillation of rhizomes gave calamus oil (1.7% w/w), which after column chromatography on a silica gel column with hexane/ethyl acetate (99:1 to 90:10) provided 1 (82% w/w) as pale yellow liquid (Rf 0.39 on silica gel TLC plate in 4% ethyl acetate in hexane), and its spectral data agreed well with reported1,13 literature values.
Preparation of 2,4,5-Trimethoxycinnamaldehyde (3) from Beta-Asarone (2)
A mixture of 2 (2.08 g, 0.01 mol) and DDQ (4.54 g, 0.02 mol) in wet dioxane (40 mL) was stirred for 15 min. A catalytic amount of silica gel (0.2-0.3 g) was added to the above mixture with constant stirring at room temperature overnight. The precipitated hydroquinone (DDQH2) was filtered and further washed with dioxane. The filtrate and washings were concentrated to dryness, resuspended in CHCl3 (25 mL), washed with H2O (2×10 mL), NaHCO3 (10%, 2×10 mL), and brine (2×10 mL), and dried over anhydrous Na2SO4. The residue obtained on evaporation of the solvents was column chromatographed on silica gel containing some neutral alumina at the top. The column was eluted with hexanes-ethyl acetate (9:1 to 3:2). The fractions were monitored on a TLC plate, and the desired fractions were combined and solvent removed under vacuum to afford 3 (1.38 g) in 62% yield as a yellow solid with Rf 0.67 (25% ethyl acetate in hexane): mp 139°C (lit.8 140°C).
Preparation of 2,4,5-Trimethoxycinnamyltosylhydrazone (4)
The cinnamaldehyde 3 (1.11 g, 0.005 mol) was dissolved in boiling MeOH (40 mL), and the solution was cooled to room temperature. p-Toluenesulfonylhydrazine (1.12 g, 0.006 mol) was added and the solution stirred at room temperature overnight. The red viscous material obtained on evaporation of solvents was chromatographed on a silica gel column with hexanes-ethyl acetate (9:1 to 3:7) as the eluent. The fractions containing 4 were pooled on the basis of TLC. Evaporation of solvent gave yellow crystals (1.54 g) of tosylhydrazone (4) in 79% yield with Rf 0.32 (25% ethyl acetate in hexane): mp 155-168°C.
Preparation of gamma-Asarone (1)
A solution of tosylhydrazone 4 (0.78 g, 0.002 mol) in glacial acetic acid (8 mL) was added dropwise to a precooled solution of sodium borohydride (0.38 g, 0.01 mol) and acetic acid (3 mL) under nitrogen atmosphere. The reaction mixture was stirred initially at 5-10°C for 1 h and finally at 90-120°C for 12 h. The mixture was poured on to ice-cooled H2O and extracted with CH2Cl2 (20 mL × 3). The organic layers were combined, washed with dilute sodium hydroxide and saturated brine, and dried over anhydrous Na2SO4. The crude product was chromatographed on silica gel (hexanes-ethyl acetate from 99:1 to 90:10) to obtain 0.18 g (43%) of 1 as a viscous liquid with Rf 0.39 (4% ethyl acetate in hexane): On the basis of the spectral data and comparing with reported literature,1,6c the liquid was identified as 1.